Cleanroom News
Masks, Microbes, and Mushrooms – How Are You Protecting Against Fungi in the Cleanroom?
We recently revisited the subject of fungal and microbial pathogens in contamination-controlled environments such as cleanrooms and were pleasantly excited to note the significant degree to which the topic sparked discussion. One of the main threads within our community concerned the selection and application of the most effective disinfectants to thwart the advances of the fungal interlopers, banishing them from areas in which they had no right to be. So this week we’re focusing on a reprise of the topic, examining what kinds of fungus can be found in the cleanroom, how to decide which products are most effective in neutralizing them, and the best practice guidelines for using these chemicals to maximum impact. Let’s dive right in – starting with a refresher on the potential lethality of fungal contamination…
Back in 2012, a now notorious incident of contamination occurring in the New England Compounding Center (NECC) which led to the deaths of 64 and the sickening of more than 800 in 20 states across the nation. We wrote extensively about this incident in our earlier article ‘Compounding Pharmacies: Exploring the Insanitary/Unsanitary Conundrum’ and it served to underscore the vital importance of protecting public health from pathogens that are often easy to overlook. Where other vectors of contamination such as microbes from decay, spoilage, or the presence of pests can be somewhat readily identified and traced to a source, the kinds of fungal spores that led in the case of NECC to a tragic outbreak of meningitis may be harder to detect. As we know from a primer published by Pharmaceutical Online, ‘Microorganisms are present in most habitats. This includes soil, water, plants, animals, hot springs, the deep sea, food, beverages, skin, and hair.’(1) And inasmuch as they are ubiquitous, they are also resilient and easy to spread both directly and indirectly. But what are they exactly? In a paper published in the Journal of Applied Microbiology, lead author R. Vijayakumar describes the organisms as follows: ‘Fungi (moulds (sic) and yeasts) are an important group of micro‐organisms. Fungi are responsible for various infections especially with the immunocompromised host. In addition to their medical importance, these organisms are associated with contamination of surfaces and spoilage of pharmaceutical, cosmetic and food products. Fungal contamination of pharmaceutical products can cause not only serious economic losses to the manufacturer but can also lead to serious health problems to customers.’(2) Indeed, this final point is one we noted above.
In order to be able to combat the potential impact of fungal contaminations, it’s important to understand a little of their microbiology and, through that, of their reach.
Of the approximately 100,000 species, only around 200 are pathogenic, according to an article published in R&D World. Characterized as ‘eukaryotic’ (a cellular organism with organelles and a nucleus protected by a membrane), molds and yeasts are the two most significant groups of fungi in terms of potential cleanroom contamination. Molds tend to grow in clusters of interconnected filaments, reproduce by generating spores, and are characterized by their ‘cottony, woolly, fluffy, or powdery aerial growths above the culture medium.’(3) Conversely, yeasts are much less companionable members of the fungal kingdom, preferring to grow as single cells that ‘bud’ asexually, producing ‘opaque, creamy, or pasty colonies.’(4) And whether mold or yeast, fungal spores range in size from 1–50 µm. For context, 1 µm (micron) is equal to 1×10−6 and that perennial unit of measurement, the human hair, measures a single strand as being between 50 and 100 microns in width. In short, fungal spores are really rather diminutive.
But what they may lack in size, they can more than make up for in effect. Although Vijayajumar et al note that ‘[t]he majority of the fungal contamination product recalls are due to contamination by hyaline fungi such as Aspergillus and Fusarium,’ these are neither the only nor yet the most powerful fungi to enter the cleanroom.(5) According to the Parenteral Drug Association (PDA), the intrusion of mold can be especially difficult to prevent and to combat: ‘Surface contaminants can become airborne, so the source could be foot- or wheel-borne contamination brought in. Inadequate storage of monitoring equipment and inadequate wipe-down procedures can be another reason. Monitoring equipment without a HEPA-filtered exhaust is known to be a contamination source. Growth of mold in walls after leaks, compromised HEPA filters, or mold growing on seals can cause airborne mold recovery. It is also important to track the mold to its source and map the transport into the area where it was recovered. Often dead spaces (e.g., in cleanrooms where the air is not cleared due to the location of HEPA filters), returns, and cleanroom and barrier system integration may allow the contaminants to linger for a long time.’(6)
Moreover, if you dismissed fungal contamination as a problem for small-scale, perhaps even a little ‘shady,’ corporate cleanrooms, it may be time to reconsider. In 2018, for example, it was found that an organization as large, and indeed venerable, as NASA was found to have issues with the presence of microbes. According to an article published in Science, it was discovered that ‘a clean room storing meteorite samples at NASA’s Johnson Space Center (JSC) in Houston, Texas, may not be quite so clean, after all. It’s contaminated with an abundance of terrestrial fungus. The microbes—most from the common genus Penicillium—could confound the search for life in off-world specimens, just as the lab is preparing to receive samples from Mars and the carbon-rich asteroid Bennu.’(7) The storage area itself was designed as an ISO class 6 facility which makes it perhaps all the more troubling that, given the degree of attention devoted to minimizing terrestrial contamination on missions, the future home of extra-planetary samples could ultimately harbor contaminants.
Perhaps like the rest of us the space agency could revisit the topic of efficient control strategies to prevent the introduction and spread of fungal and microbial contamination within a controlled setting. According to Pharmaceutical Online, some of the most basic best practices of aseptic techniques include ensuring that sterile items are neither exposed to not come into contact with non-sterile items or environments, ‘[n]ever breaking first air or reaching over exposed product, components, or fill lines,’ and always using sterile components and appropriate personal protection equipment (PPE) such as non-shedding gowns and snugly-fitting gloves.(8) As you’ll note, the emphasis is placed on minimizing the impact of the employees as vectors of contamination. And this is because, as humans, ‘we are expected to be carriers of some unwanted microorganisms. In fact, over 200 different species of bacteria are associated with humans and are found in the intestines, eyes, nose, mouth, hair, and skin. Believe it or not, dry skin can contain thousands of microbes per square millimeter! People are not only a source of contamination, but also an agent for transferring contamination to locations that could pose a risk to [a] product.’(9) Of course, cleanroom staff are not willing vectors of transmission but nonetheless ‘[h]uman error in the cleanroom can also be attributed all the way back to the locker rooms, where operators gather prior to going into the gowning area. It is here that the operators need to ensure proper hand washing and sanitizing. The firm should have a standard operating procedure that lists their (sic) hygiene and cleanliness standards.’(10) Furthermore, these SOPs should prevent against the intrusion of fungi into the environment from the multitude of other sources such as ‘kick plates, bags, boxes, markers, intervention equipment, cart wheel, ceiling tiles, poorly maintained flooring and in some cases, high‐pressure impingement application devices for applying germicides.’(11)
But assuming the worst what’s the next step? That’s easy: take decisive action.
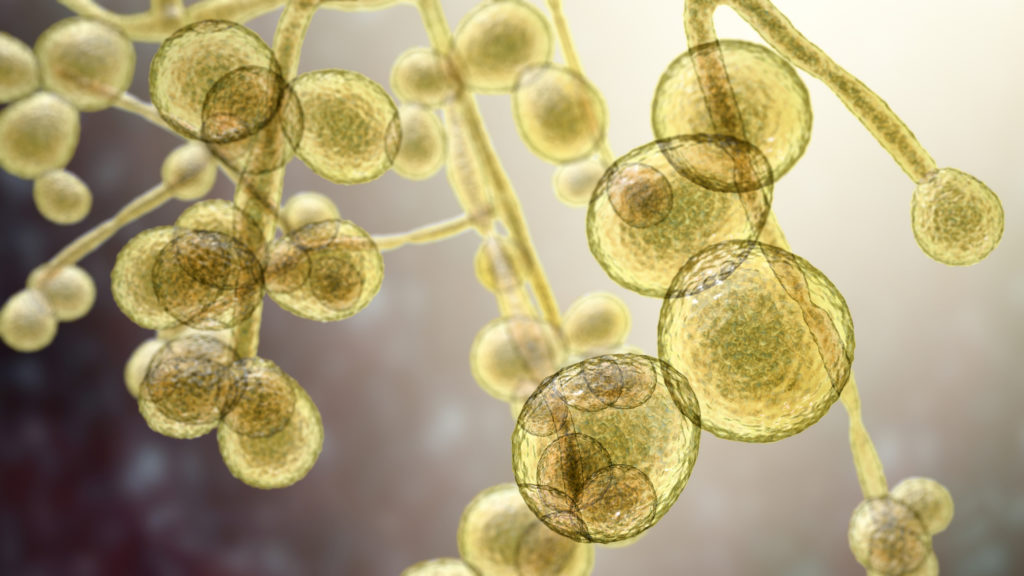
The presence of fungal spores in a controlled environment is, of course, an indication that strategies have failed at the preventative level and, as such, steps to neutralize the problem become mandatory. There are, of course, an oftentimes bewildering array of products for disinfecting, sterilizing, sanitizing, and general cleaning tasks, and the United States Pharmacopeia (USP) <1072> is a solid source of information on disinfectants and antiseptics. According to the USP guidelines, although the basic effectiveness of a disinfecting solution is determined by its own properties – pH, concentration, and strength, for example – other factors must also be considered. When it comes to sanitation, the type of surface, degree of organic matter present, and the type/number of contaminants present all play significant roles in the success or otherwise of the sanitation process. Moreover, questions of application and technique are of some importance as products have specific guidelines regarding contact time – that is, the ‘wet time’ in which a product is active upon a cleaning surface – and this can change depending on the contaminant. Furthermore, the application method used – spraying, wiping et cetera – makes a difference in efficacy as does, of course, the knowledge and diligence of the technician. Finally, there are also environmental factors to consider such as the ambient temperature, humidity, airflow, the soil type from which the fungal spores/particulates originated, the quality of water (hard or soft?), and the overall bioburden.
A recently published article in Cleanroom Technology examines the question of microbial contamination, disinfectant testing, the responsibilities of manufacturing facilities and regulatory agencies, and the on-going nature of processes designed to maintain a contamination-free cleanroom environment. We recommend taking a look at it here. Equally, with the broad range of options available, we also suggest reviewing Berkshire’s own products where you can now drill down by industry to find the most appropriate items for your own cleanroom. From masks to mats to mops, socks to swabs, it is likely we have the equipment you need. ‘We understand the challenge of choosing the appropriate application-specific consumable and how important that decision is in maintaining a contamination-free cleanroom environment.’ notes Jamie Haskins, Global Director, Marketing.
Of course that’s not the end of the story. Even when the surfaces have been sanitized, it is critical to be able to evaluate the efficacy of the procedures. With this in mind, Gerbig – a provider of modular cleanrooms, workstations, pass-thru units, and cleanroom testing & certification, based in Minneapolis, MN – recommends two methods of in-house testing and subsequent analysis to ensure the fullest remediation possible. The complementary, dual testing approach focuses on both the solution used in cleaning and the surface to be sanitized, performing both suspension tests and carrier tests, with an on-going statistical comparison. Let’s take a closer look…
According to Gerbig’s article, suspension tests help determine the most appropriate disinfectant product to use in a given situation. In suspending a fungal sample in an ‘appropriate dilution of the test disinfectant’ at room temperature, the test reveals the time taken to achieve a significant reduction in the number of microorganisms.(12) Carrier tests, on the other hand, focus on the material of the surface itself – glass, stainless steel, vinyl, etc., to pinpoint the correct product for use. In terms of the statistical comparisons of these two sets of results, Gerbig recommends performing repeated tests in the target environment throughout the year in order to account for seasonal changes in types and volumes of fungal/microbial particles. In short, the battle against fungal intrusion into the cleanroom is a continuous one – but, as the NECC tragedy showed, it is not one in which we can afford to be delinquent.
Granted, it’s an uphill battle but, with the correct information based in solid science, it’s one we can fight. If, after reading through some of the articles and texts we’ve already mentioned, you are looking for a next step the Association of Official Analytical Collaboration International, now better known as AOAC INTERNATIONAL, offers an Official Methods of AnalysisSM (OMA) program in which approved methods and consensus standards are scrutinized to ensure optimal standards. The organization addresses the fields of microbiology, chemistry, and molecular biology, amongst others, and its methods are ‘recognized in the U.S. Code of Federal Regulations and are legally defensible in court worldwide.’(13) For those with an interest in leveraging some of the more than 3,000 methods validated by the AOAC, some of which have been adopted by organizations such as the International Organization for Standardization (ISO), the International Union of Pure and Applied Chemistry (IUPAC), and the Codex Alimentarius Commission, the OMA is a good place to start. Happy reading!
Fungus among us? How is your cleanroom preventing infestation and contamination by some of nature’s smallest pathogens? Are you worried? We’d love to know your thoughts!
References:
- https://www.pharmaceuticalonline.com/doc/cleanroom-microbiology-identifying-controlling-sources-of-contamination-0001
- https://sfamjournals.onlinelibrary.wiley.com/doi/full/10.1111/jam.12888
- https://www.rdworldonline.com/control-strategies-for-fungal-contamination-in-cleanrooms/
- ibid
- https://sfamjournals.onlinelibrary.wiley.com/doi/full/10.1111/jam.12888
- https://www.pda.org/pda-letter-portal/home/full-article/the-moldy-nightmare-questions-and-answers-part-1
- https://www.sciencemag.org/news/2018/03/nasa-clean-room-contaminated-fungus
- https://www.pharmaceuticalonline.com/doc/cleanroom-microbiology-identifying-controlling-sources-of-contamination-0001
- ibid
- https://www.pharmaceuticalonline.com/doc/best-practices-for-keeping-human-error-out-of-the-cleanroom-0001
- https://sfamjournals.onlinelibrary.wiley.com/doi/full/10.1111/jam.12888
- https://www.gerbig.com/how-to-control-fungus-in-cleanrooms/
- https://www.aoac.org/scientific-solutions/standards-and-official-methods/

















Pingback: Masks, Microbes, and Mushrooms – How Are You Protecting Against Fungi in the Cleanroom? - Cleanroom News | Berkshire Corporation