Berkshire Products, Knowledge Base
Powerful Wetting Solutions: The Soaring Popularity of IPA
In last week’s article, Unlocking the Best Pre-Wetted Wiper – Part 2, we shared some insight about essential cleaning requirements to consider in the cleanroom. And we touched on the relevance of IPA (Isopropyl Alcohol) concentration when choosing a pre-wetted wiper for your cleanroom needs. That’s why this week, we will dive a bit deeper and explore the liquids incorporated in packaged pre-wetted wipers. But, first, it’s essential to understand why an isopropyl alcohol-water mixture is a predominant liquid used to dampen wipers.
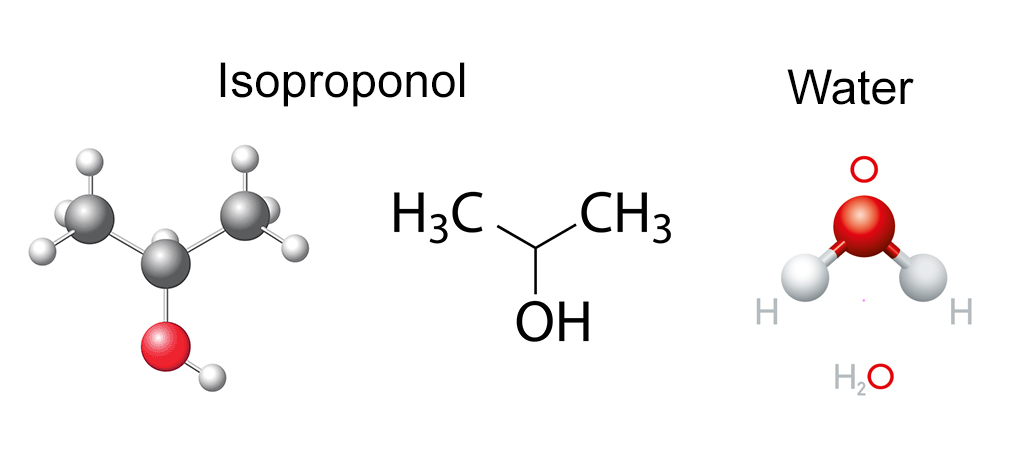
First, you can mix IPA and water in all proportions without separating phases – e.g., 1% IPA in water to 1% water in IPA.
Second, IPA and water can be highly purified for use in the cleanroom, leaving virtually no residue.
Third, IPA provides both polar and non-polar characteristics. It does not significantly inhibit the dissolution of ionic salts (the polar aspect) and can dissolve small amounts of hydrocarbon soils (the non-polar part). So, the more significant the proportion of water in the mixture, the greater is the polar characteristic.
Fourth, other alcohols[1] such as methanol and ethanol have an insufficient non-polar character to be effective against greasy soils. And alcohols such as butanol and pentanol have too much non-polar character to provide unrestricted miscibility with water. For example, a 10% mixture of butanol in water (volume/volume) is the highest concentration of butanol in water that can be tolerated before the separation of the butanol and water occurs.
So, the concept that “IPA is just right” is not a fairy tale.
However, when choosing pre-wetted wipers with IPA, it is also critical to consider flammability characteristics.
For example, a pre-wetted wiper with mixtures containing 9% IPA or less has a designated rating of “combustible,” not “flammable.” And you can store pre-wetted wipers containing 9% IPA or less without issue. They also would be recommended for required routine cleaning of lightly-soiled environmental surfaces since pre-wetted wipers containing 9% IPA or less don’t need special storage and can be accessed easily within the cleanroom.
However, pre-wetted wipers containing higher IPA percentages with a flammable rating require appropriate storage. Therefore, they must be stored in a proper safety cabinet.
Since pre-wetted wipes with a higher concentration of IPA are better where surface soils are of concern, or there is some required disinfection capability, we would recommend the 70% IPA pre-wetted product.
It’s also essential to consider, from an environmental perspective, the contribution of volatile organic compounds (VOCs). Why is this an important aspect to consider? Because VOC contribution is a function of IPA concentration. So, the more IPA in the mixture, the higher the VOC contribution.
Accordingly, if low-concentration IPA pre-wetted products can accomplish the necessary cleaning operation, they would be a viable option over a higher concentration IPA. But again, there are multiple considerations when choosing the best pre-wetted wiper for your cleanroom needs.
But what if IPA isn’t an option for your industry’s surface disinfection needs? Are there inorganic wetting solutions with no flammability or VOC issues? The answer is yes. Commercial pre-wetted wipers containing hydrogen peroxide or sodium hypochlorite are available for the health care industry for surface disinfection. And these wetting solutions are inorganic and have no flammability or VOC issues.
We hope we’ve provided some additional insight into why an isopropyl alcohol-water mixture is a predominant liquid used to dampen wipers and why the IPA concentrations matter – not only for cleaning requirements but also for safe storage.
Of course, we’re always happy to answer your questions regarding your cleanroom contamination needs, so feel free to reach out to our trained specialists for more information.
We hope you’ll join us next week when we share some insight about optimum wiping techniques for cleaning.
1The alcohols described have the following number of carbon atoms per molecule
Methanol – 1; Ethanol – 2; IPA – 3; Butanol – 4; Pentanol – 5. As the number of carbon atoms in the molecule increases, the non-polar character of the molecule increases. Water is a polar solvent that can dissolve all proportions of small alcohols such as methanol, ethanol or IPA. For butanol and the higher alcohols the solubility becomes limited. Think oil and water.

















HAVE AN IDEA FOR CONTENT?
We are always looking for ideas and topics to write about.
Contact Us